‘Surface Chemistry Class 12 Best Notes ‘ PDF Quick download link is given at the bottom of this article. You can see the PDF demo, size of the PDF, page numbers, and direct download Free PDF of ‘Surface Chemistry Notes’ using the download button.
Surface Chemistry Handwritten Notes PDF Free Download
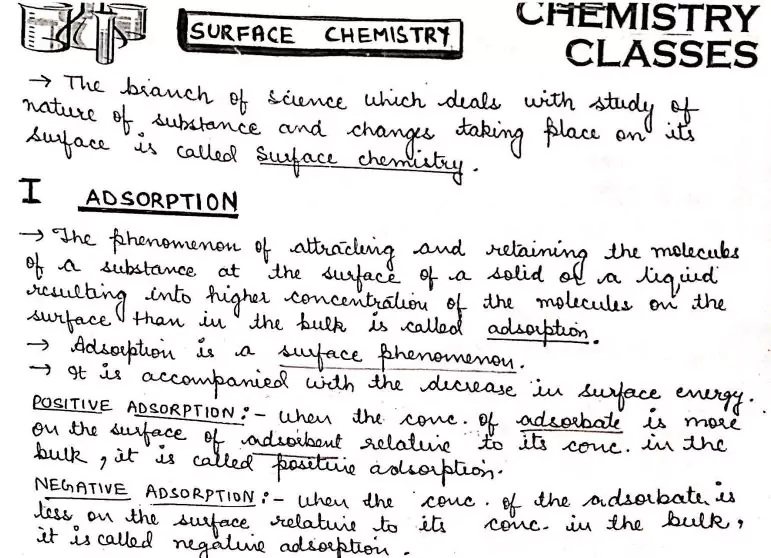
Surface Chemistry
Surface chemistry deals with phenomena that occur at the surfaces or interfaces. The interface or surface is represented by separating the bulk phases by a hyphen or a slash.
For example, the interface between a solid and a gas may be represented by solid gas or solid/gas. Due to complete miscibility, there is no interface between the gases.
The bulk phases that we come across in surface chemistry may be pure compounds or solutions.
The interface is normally a few molecules thick but its area depends on the size of the particles of bulk phases.
Many important phenomena, noticeable amongst these being corrosion, electrode processes, heterogeneous catalysis, dissolution, and crystallization occur at interfaces.
The subject of surface chemistry finds many applications in industry, analytical work, and daily life situations.
To accomplish surface studies meticulously, it becomes imperative to have a really clean surface.
Under a very high vacuum of the order of Pascal, it is now possible to obtain an ultra-clean surface of the metals.
Solid materials with such clean surfaces need to be stored in a vacuum otherwise these will be covered by molecules of the major components of air namely dioxygen and dinitrogen.
In this Unit, you will be studying some important features of surface chemistry such as adsorption, catalysis, and colloids including emulsions and gels.
Surface Chemistry After studying this Unit, you will be able to
• describe the interfacial phenomenon and its significance;
• define adsorption and classify it into physical and chemical adsorption;
• explain the mechanism of adsorption;
• explain the factors controlling adsorption from gases and solutions on solids;
• explain adsorption results on the basis of Freundlich adsorption isotherms;
• appreciate the role of catalysts in the industry;
• enumerate the nature of the colloidal state;
• describe the preparation, properties, and purification of colloids;
• classify emulsions and describe their preparation and properties;
• describe the phenomenon of gel formation;
• list the uses of colloids.
Objectives
Some of the most important chemicals are produced industrially by means of reactions that occur on the surfaces of solid catalysts.
There are several examples, which reveal that the surface of a solid has the tendency to attract and retain the molecules of the phase with which it comes into contact.
These molecules remain only at the surface and do not go deeper into the bulk. The accumulation of molecular species at the surface rather than in the bulk of a solid or liquid is termed adsorption.
The molecular species or substance, which concentrates or accumulates at the surface is termed adsorbate and the material on the surface on which the adsorption takes place is called adsorbent.
Adsorption is essentially a surface phenomenon.
Solids, particularly in finely divided states, have a large surface area, and therefore, charcoal, silica gel, alumina gel, clay, colloids, metals in finely divided states, etc. act as good adsorbents.
Adsorption in action
(i) If a gas like O2, H2, CO, Cl2, NH3or SO2 is taken in a closed vessel containing powdered charcoal, it is observed that the pressure of the gas in the enclosed vessel decreases.
The gas molecules concentrate at the surface of the charcoal, i.e., gases are adsorbed at the surface.
(ii) In a solution of an organic dye, say methylene blue, when animal charcoal is added and the solution is well shaken, it is observed that the filtrate turns colorless.
The molecules of the dye, thus, accumulate on the surface of the charcoal, i.e., are adsorbed.
(iii) Aqueous solution of raw sugar, when passed over beds of animal charcoal, becomes colorless as the coloring substances are adsorbed by the charcoal.
(iv) The air becomes dry in the presence of silica gel because the water molecules get adsorbed on the surface of the gel.
It is clear from the above examples that solid surfaces can hold gas or liquid molecules by virtue of adsorption.
The process of removing an adsorbed substance from a surface on which it is adsorbed is called desorption.
| Author | – |
| Language | English |
| No. of Pages | 36 |
| PDF Size | 15 MB |
| Category | Chemistry |
| Source/Credits | drive.google.com |
Related PDFs
Surface Chemistry Handwritten Notes PDF Free Download
