‘Atoms Notes And Important Topics For Boards’ PDF Quick download link is given at the bottom of this article. You can see the PDF demo, size of the PDF, page numbers, and direct download Free PDF of ‘Class 12 Physics Handwritten Notes Chapter 12’ using the download button.
Atom And Nuclei Handwritten Notes For 12th Class Physics PDF Free Download
Atoms: Introduction
By the nineteenth century, enough evidence had accumulated in favor of the atomic hypothesis of matter.
In 1897, the experiments on electric discharge through gases carried out by the English physicist J. J. Thomson (1856 –1940) revealed that atoms of different elements contain negatively charged constituents (electrons) that are identical for all atoms.
However, atoms as a whole are electrically neutral. Therefore, an atom must also contain some positive charge to neutralize the negative charge of the electrons.
But what is the arrangement of the positive charge and the electrons inside the atom?
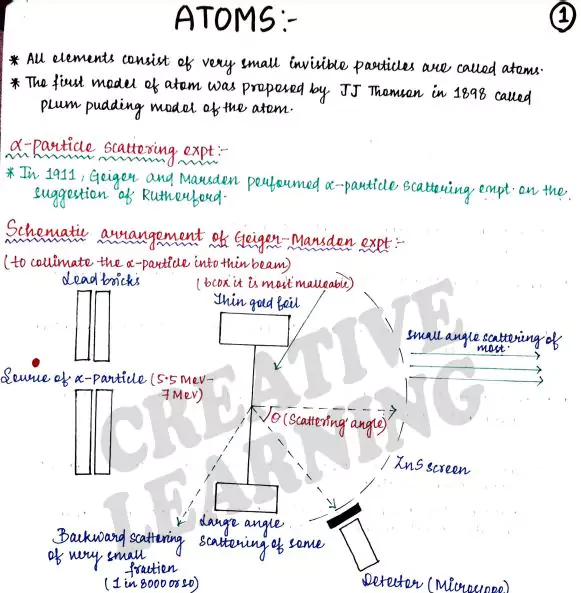
In other words, what is the structure of an atom? The first model of the atom was proposed by J. J. Thomson in 1898.
According to this model, the positive charge of the atom is uniformly distributed throughout the volume of the atom and the negatively charged electrons are embedded in it like seeds in a watermelon.
This model was picturesquely called the plum pudding model of the atom.
However subsequent studies on atoms, as described in this chapter, showed that the distribution of the electrons and positive charges are very different from that proposed in this model.
We know that condensed matter (solids and liquids) and dense gases at all temperatures emit electromagnetic radiation in which a continuous distribution of several wavelengths is present, though with different intensities.
This radiation is considered to be due to oscillations of atoms Chapter Twelve ATOMS.
Atoms and molecules are governed by the interaction of each atom or molecule with its neighbors.
In contrast, light emitted from rarefied gases heated in a flame, or excited electrically in a glow tube such as the familiar neon sign or mercury vapor light has only certain discrete wavelengths.
The spectrum appears as a series of bright lines. In such gases, the average spacing between atoms is large.
Hence, the radiation emitted can be considered due to individual atoms rather than because of interactions between atoms or molecules.
In the early nineteenth century, it was also established that each element is associated with a characteristic spectrum of radiation, for example, hydrogen always gives a set of lines with a fixed relative position between the lines.
This fact suggested an intimate relationship between the internal structure of an atom and the spectrum of radiation emitted by it.
In 1885, Johann Jakob Balmer (1825 – 1898) obtained a simple empirical formula that gave the wavelengths of a group of lines emitted by atomic hydrogen.
Since hydrogen is the simplest of the elements known, we shall consider its spectrum in detail in this chapter.
Ernst Rutherford (1871–1937), a former research student of J. J. Thomson, was engaged in experiments on α-particles emitted by some radioactive elements.
In 1906, he proposed a classic experiment of scattering of these α-particles by atoms to investigate the atomic structure.
This experiment was later performed around 1911 by Hans Geiger (1882–1945) and Ernst Marsden (1889–1970, who was a 20-year-old student and had not yet earned his bachelor’s degree).
The details are discussed in Section 12.2. The explanation of the results led to the birth of Rutherford’s planetary model of the atom (also called the nuclear model of the atom).
According to this the entire positive charge and most of the mass of the atom is concentrated in a small volume called the nucleus with electrons revolving around the nucleus just as planets revolve around the sun.
Rutherford’s nuclear model was a major step toward how we see the atom today. However, it could not explain why atoms emit light of only discrete wavelengths.
How could an atom as simple as hydrogen, consisting of a single electron and a single proton, emit a complex spectrum of specific wavelengths?
In the classical picture of an atom, the electron revolves around the nucleus much like the way a planet revolves around the sun.
However, we shall see that there are some serious difficulties in accepting such a model.
| Author | – |
| Language | English |
| No. of Pages | 10 |
| PDF Size | 5 MB |
| Category | Science |
| Source/Credits | drive.google.com |
Related PDFs
Atom And Nuclei Handwritten Notes For 12th Class Physics PDF Free Download
