‘Ligands And Charges Class 12’ PDF Quick download link is given at the bottom of this article. You can see the PDF demo, size of the PDF, page numbers, and direct download Free PDF of ‘Ligands And Charges’ using the download button.
List of Ligands And Their Names PDF Free Download
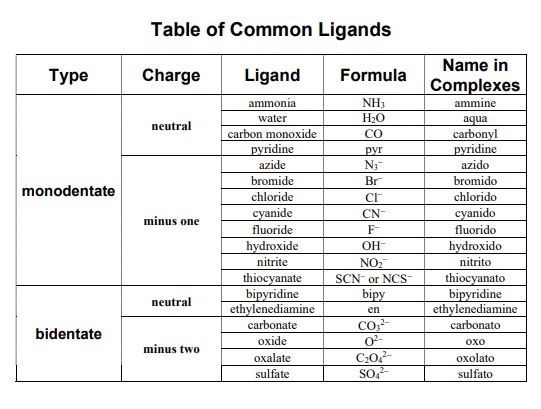
List Of Ligands And Charges
WERNERS’ COORDINATION THEORY
Coordination compounds were known in the eighteenth century.
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. Bonding with a metal generally involves a formal donation of one or more electron pairs to the ligand.
The nature of the metal-ligand bond can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases involving Lewis acidic “ligands” are known.
Metals and metalloids are bound to the ligand under almost all conditions, although gaseous “naked” metal ions can be produced in a high vacuum.
Ligands in a complex determine the reactivity of the central atom, including the ligand substitution rate, the reactivity of the ligand itself, and redox.
Ligand selection is an important consideration in many practical fields, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemistry.
It was a mystery for the chemist, of those days to understand why a stable salt like CoCl3 reacts with varying numbers of stable molecules or compounds such as ammonia to give several new compounds: CoCl3.6NH3, and CoCl3.5NH3 andCoCl3.4NH3; and what are their structures?
These compounds differed from each other in their chloride ion reactivity. Conductivity measurements on solutions of these compounds showed that the number of ions present in the solution for each compound is different.
Several theories were proposed, but none could satisfactorily explain all the observable properties of these compounds and similar other series of compounds that had been prepared by then.
It was only in 1893 that Werner put forward a set of ideas which are known as Werner’s coordination theory, to explain the nature of bonding in complexes.
His theory has been a guiding principle in inorganic chemistry and the concept of valence.
The important postulates of Werner’s theory are:
Metals exhibit two types of valence:
(a) Primary valence (ionizable)
(b) Secondary valence (non-ionizable).
Primary or ionizable valence is satisfied by negative ions and corresponds to the oxidation state of the metal.
- The secondary or non-ionizable valence, which is satisfied by negative, positive, or neutral groups, is equal to the coordination number of the metal ions.
- Every metal tends to satisfy both its primary and secondary valence.
- The secondary valence is directed toward fixed positions in space i.e. this has spatial arrangement corresponding to different coordination numbers.
- For the complexes, CoCl3.6NH3 CoCl3.5NH3, and CoCl3.4NH3, the number of ionizable ions in these complexes is three, two, and one, respectively.
- It has been proved by precipitation reactions and conductivity measurements.
- On the basis of Werner’s postulate these compounds are formulated as:
- The cation is named before the anion, as in other ionic compounds.
- The rule holds regardless of whether the complex ion bears a net positive or a negative charge.
- For example, in K3[Fe(CN)6] and [Co(NH3)4Cl2]Cl compound, we name the K+ and [Co(NH3)4Cl2] first, respectively.
- Within a complex ligands are named first, in alphabetical order, and the metal ion is named last.
- The name of an anionic ligand ends with the letter ‘O’, whereas a neutral ligand is usually called by the name of the molecule.
- The exceptions are H2O (aqua), CO (carbonyl), and NH3 (ammine).
| Author | – |
| Language | English |
| No. of Pages | 01 |
| PDF Size | 2 MB |
| Category | PDF of Lists |
| Source/Credits | Drive.google.com |
List of Ligands And Their Names PDF Free Download
